Answer: The unbalanced chemical equation is written below.
Step-by-step explanation:
Unbalanced chemical equation does not follow law of conservation of mass.
In an unbalanced chemical equation, total number of individual atoms on the reactant side will not be equal to the total number of individual atoms on the product side.
The chemical equation for the reaction of diboron trioxide and magnesium metal follows:
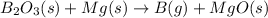
Hence, the unbalanced chemical equation is written above.