Answer:
Mass of He required = 8.0 g
Step-by-step explanation:
Given,
Initial moles of He = 2.0 mol
Initial pressure = 1.00 atm
final pressure = 2.00 atm
Ideal gas equation,
PV = nRT
As V, R and T are constant
So,
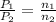
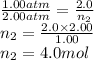
Molar mass of He = 4.00 g/mol
No. of moles of He needs to be added = 4.0 - 2.0 = 2.0 mol
Mass = No. of mole × Molar mass
= 2.0 × 4.0
= 8.0 g