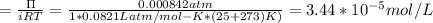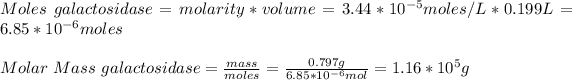Answer:
Molecular mass of β‑galactosidase = 1.16*10^5g
Step-by-step explanation:
The osmotic pressure (π) is related to the molarity (M) of a solution by the following equation:
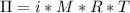
i = Von't hoff factor = 1 for non-electrolytes
R = gas constant = 0.0821Latm/mol-K
T = temperature
Based on the above equation, molarity of β‑galactosidase is: