Answer:
ΔG for the given system is 28.421 kJ. The system is non spontaneous.
Step-by-step explanation:
ΔH = 147 kJ =147,000 J= Enthalpy of the system
ΔS = -67.0 J/K = Entropy change
T = 149 °C = 422.15 K = Temperature of the system
((T)°C=273.15 +T K)
ΔG = Gibbs free energy ?
The Gibbs free energy 's expression is given by:
ΔG = ΔH - T ΔS
- ΔG > 0 , non spontaneous
- ΔG < 0, spontaneous
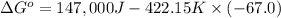
ΔG = 28,421 J = 28.421 kJ
Since, value of Gibbs free energy is positive this means that reaction is non spontaneous.