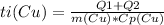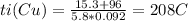Answer:
208 C
Step-by-step explanation:
First we need the specific heat capacity of copper.

The specific heat capacity for ice is:
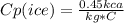
And the latent heat of melting ice into water is:
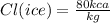
Since the container has water and ice together, they must be at 0 C (assuming normal atmospheric pressure), and the copper piece too.
Therefore, all the ice heated from -17C to 0C. For this it took an energy of
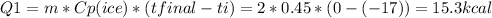
Also, part of the ice melted into water, this consumed energy too:
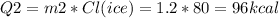
The copper piece provided that heat, it released it by cooling down.
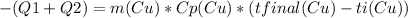
The copper ended at 0C.