Answer:
0.44 cal/(g C)
Step-by-step explanation:
I assume the calorimeter is adiabatic.
First I need the specific heat capacity of iron, this is:
Cp(Fe) = 0.11 cal/(g * C)
Both bodies in the calorimeter will exchange heat until they reach equilibrium at the final temperature. The heat exchanged by each body is:
Q = m * Cp * (tfin - ti)The iron will be giving off heat (negative heat) and the liquid receiving it (positive heat, so:
-m(Fe) * Cp(Fe) *(tfin - ti(Fe)) = m(liq) * Cp(liq) * (tfin - ti(liq))
Rearranging:
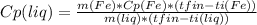
So:
