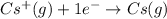Answer : The correction equation will be:
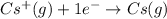
Explanation :
Electron affinity : It is defined as the addition of electron to the atom or to the ion. The atom or ion is always in gaseous phase.
In other words we can say that, the gaining of electrons is said to be an electron affinity.
As, the given element is a metal given in cationic form, so its positive ion will gain an electron to form a neutral atom.
The balanced and correct equation for electron affinity of the cesium ion will be: