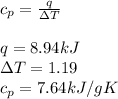Answer:
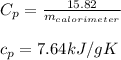
Step-by-step explanation:
First we set the equation for the combustion:
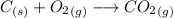
As the equation is already balanced, we just need to look at the coefficients, that are 1 for all the elements of the reaction, so 1 mole of carbon produces 1 mole of carbon dioxide. Now we need to find how many moles are 0.5662 g of carbon:
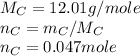
Know we proceed to know how many moles of carbon dioxide were produced. As we know the reaction is 1:1, the moles of carbon dioxide are 0.047.
Now we need to know the mass of the carbon dioxide produced:
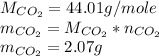
From literature we find that the enthalpy of formation of carbon dioxide is -393.5 kJ/mole. We need it in terms of mass, so we convert it with the molar mass as follows:
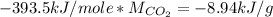
With this we can continue with the calculation given the equation Q=q*m
So Q=18.51 kJ
To calculate the heat capacity we use Q=mCpΔT, therefore:
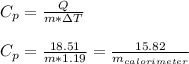
As we don't know the mass of the calorimeter, we just leave the specific heat capacity as follows: