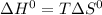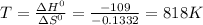Answer:
Temperature = 818 K
Step-by-step explanation:
The given reaction is:
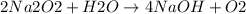
The change in enthalpy is: ΔH°= -109 kJ
The change in entropy is : ΔS° = -133.2 J/k = -0.1332 kJ/K
Based on the Gibbs-Helmholtz equation the standard free energy change is given as:
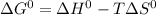
At equilibrium ΔG° = 0. therefore: