Step-by-step explanation:
The given data is as follows.
mass of sample (X) = 0.740 g,
 =
=
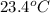
 =
=
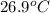
As, Density =

1.00 g/ml =
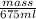
mass = 675 g
Hence, weight of water = 675 g and heat capacity is given as 534
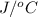
Heat produced by combustion of TNT = Heat gained by the water
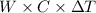 =
=
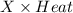
Heat =
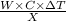
=
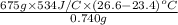
= 1704831 J
As 1 kJ = 1000 J. So, 1704831 J = 1704.831 kJ
Thus, we can conclude that heat produced by the combustion of the TNT sample is 1704.831 kJ.