Answer:
174,957.143 grams of potassium and 89,228.478 grams of potassium nitrate will be needed.
Step-by-step explanation:
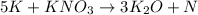
Mass of nitrogen = 27 lbs = 12,247 g
1 lbs = 453.592 g
Moles of nitrogen =
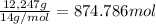
According to reaction, 1 mole of nitrogen is produced from 5 moles of potassium and 1 mole of potassium nitrate.
Then 874.786 mol of nitrogen will be obtained from :
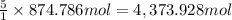 of potassium.
of potassium.
Then 874.786 mol of nitrogen will be obtained from :
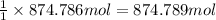 of potassium nitrate.
of potassium nitrate.
Mass of 4,373.928 moles of potassium:
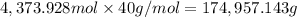 of potassium
of potassium
Mass of 874.789 moles of potassium nitrate:
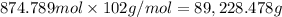 of potassium nitrate
of potassium nitrate