Step-by-step explanation:
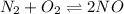
Initially Concentration(M)
0.100 M 0.500 M 0.100 M
At equilibrium(0.100-x) (0.500 -x) (0.100 +2x)
Equilibrium constant of the reaction =
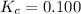
An equilibrium expression for the given reaction is given as:
![K_c=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2020/formulas/chemistry/college/h97e3v9ia7fb0kvxd3enqoq711mg7wqk69.png)
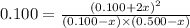
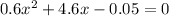
On solving this quadratic equation we get:
x = 0.01085
Equilibrium concentration of nitrogen gas"
![[N_2]= (0.100-x)=0.100 M - 0.01085 M= 0.08915 M](https://img.qammunity.org/2020/formulas/chemistry/college/kvdne74w993nfam6wckd3qdo1ql8nw3jsi.png)
Equilibrium concentration of oxygen gas"
![[O_2]= (0.500-x)=0.500 M - 0.01085 M= 0.48915 M](https://img.qammunity.org/2020/formulas/chemistry/college/6340zdey5418wtkut24whhyvpl6z0yridc.png)
Equilibrium concentration of NO gas"
![[N_2]= (0.100+2x)=0.100 M - 2* 0.01085 M= 0.1217 M](https://img.qammunity.org/2020/formulas/chemistry/college/py9id6zp9hvlzdkomjw9ewk0t7e6vg0tcc.png)