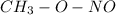Answer:
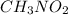 has higher boiling point
has higher boiling point
Step-by-step explanation:
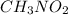 has higher boiling point because in
has higher boiling point because in
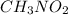 ,
,
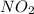 has a very strong dipole and we know that whenever there is dipole-dipole interaction then boiling point increases. On the other hand in
has a very strong dipole and we know that whenever there is dipole-dipole interaction then boiling point increases. On the other hand in
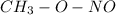 the NO group has not so strong dipole which weakens its boiling point
the NO group has not so strong dipole which weakens its boiling point
So the boiling point of
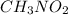 is higher than the boiling point of
is higher than the boiling point of