Answer : The equilibrium concentration of C is 4.6 M
Solution : Given,
Concentration of
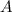 at equilibrium = 1.1 M
at equilibrium = 1.1 M
Concentration of
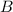 at equilibrium = 1.4 M
at equilibrium = 1.4 M

First we have to calculate the value of equilibrium constant.
The given equilibrium reaction is,
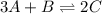
The expression of
 will be,
will be,
![K_c=([C]^2)/([A]^3[B])](https://img.qammunity.org/2020/formulas/chemistry/college/tdfwpheej0eb4g31vwot43gn1anpqjgkqq.png)
![11.3=([C]^2)/((1.1)^3* (1.4))](https://img.qammunity.org/2020/formulas/chemistry/college/lmswk0qbabv2bs8yco6d6ur4flzyc3qua3.png)
![[C]=4.6M](https://img.qammunity.org/2020/formulas/chemistry/college/39ocluh980v51e5ikrwywm9x903vwn20dd.png)
Therefore, the equilibrium concentration of C is 4.6 M