Answer: There are
a) 2.12 g of Ir-192 after 199.2 days
b) 0.52 g of Ir-192 after 350 days
Step-by-step explanation:
Radioactive decay follows a first-order kinetics since the half life does not depend on the initial amount, then the equation which describes this process is:
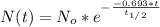
Where
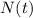 is the amount given a certain t time, and
is the amount given a certain t time, and
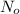 is the initial amount.
is the initial amount.
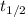 is the half life.
is the half life.
Then, the radioactive decay equation for this problem is:
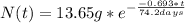
Note that the half life and the given time t must be on the same units, in this case days. Finally you calculate the amount for a) 199.2 days and b) 350 days:
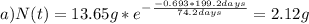
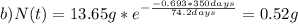
Hope it helps!