Step-by-step explanation:
As it is known that density is defined as the mass present in per unit volume.
Mathematically, Density =
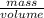
Since, density is directly proportional to mass. Hence, more is the mass of an object more will be its density.
Hence, mass of krypton is 84 whereas mass of iron is 56. This shows that mass of krypton is more than the mass of iron.
Therefore, density of krypton is more than the density of iron.