Suppose you add x liters of pure water to the 10 L of 25% acid solution. The new solution's volume is x + 10 L. Each L of pure water contributes no acid, while the starting solution contains 2.5 L of acid. So in the new solution, you end up with a concentration of (2.5 L)/(x + 10 L), and you want this concentration to be 10%. So we have
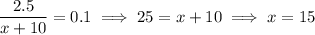
and so you would need to add 15 L of pure water to get the desired concentration of acid.