Step-by-step explanation:
In an electrochemical cell, two half electrochemical processes occur. One of which is oxidation process and other is reduction process.
Oxidation reaction is defined as the reaction in which an atom looses its electrons. The oxidation number of the atom gets increased during this reaction.
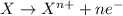
Reduction reaction is defined as the reaction in which an atom gains electrons. The oxidation number of the atom gets reduced during this reaction.
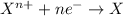
Oxidation half reaction occurs at anode and reduction half reaction occurs at cathode.
Half reaction of the overall reaction represents the two processes which occur inside the cell which is oxidation and reduction.