Step-by-step explanation:
As the first reaction equation is as follows.
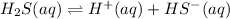 ..... (1)
..... (1)
So, expression for equilibrium constant will be as follows.
![K'_(c) = ([H^(+)][HS^(-)])/([H_(2)S])](https://img.qammunity.org/2020/formulas/chemistry/college/cgdc97xex93h2llo2hzoh7vvo3t8r5cezm.png) =
=
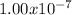
The second reaction equation is as follows.
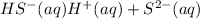 ...... (2)
...... (2)
So, expression for equilibrium constant will be as follows.
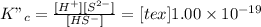
Hence, the net reaction equation will be (1) + (2) as follows.
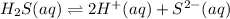
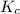 =
=
![\frac{[H^(+)]^(2)[S^(2-)]}{[H_(2)S]]()
=
![[H^(+)][HS^(-)]{[H_(2)S]](https://img.qammunity.org/2020/formulas/chemistry/college/xyu8wrx2vdio811zd7xm2s3g5vl22hakxg.png) x
x
![([H^(+)][S^(2-)])/([HS^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/dg0vydz6cpyipaa91iyjgg3783uroq06o6.png)
=
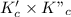
=
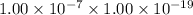
=
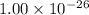
Thus, we can conclude that the equilibrium constant for the reaction:
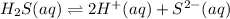 is K =
is K =
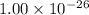 .
.