Answer: e. Two of these
Step-by-step explanation:
(a)
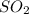
When
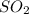 is dissolved in water. It reacts to form sulfurous acid. The chemical reaction is shown below:
is dissolved in water. It reacts to form sulfurous acid. The chemical reaction is shown below:
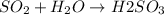
Therefore,
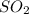 is acidic when dissolved in water.
is acidic when dissolved in water.
(b)
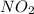
When
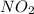 is dissolved in water. It reacts to form nitric acid. The chemical reaction is shown below:
is dissolved in water. It reacts to form nitric acid. The chemical reaction is shown below:
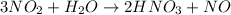
Therefore,
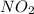 is acidic when dissolved in water.
is acidic when dissolved in water.
(c)
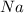
Sodium is a metal. It gives sodium hydroxide on reaction with water. The chemical reaction is as follows:
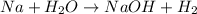
Therefore, Na is basic when dissolved in water.
(d)
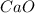
Calcium oxide is a metallic salt. It gives calcium hydroxide on reaction with water. The chemical reaction is as follows:
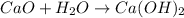 ,
,
Therefore,
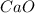 is basic when dissolved in water.
is basic when dissolved in water.
(e) Two of these.
Since both
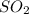 and
and
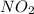 give acids when dissolved in water, both are acidic.
give acids when dissolved in water, both are acidic.