Answer:
enthalpy of water at
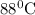 is 3394.8 kJ
is 3394.8 kJ
Step-by-step explanation:
Mass does not change with change in temperature .
Hence change in enthalpy (
 ) during increase in temperature from
) during increase in temperature from
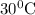 to
to
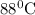 is-
is-
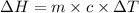
where m is mass of water, c is specific heat capacity of water and
 is change in temperature.
is change in temperature.
Here, m = 8 kg, c = 4.2 kJ/(kg.K),
 = (88-30) K = 58 K
= (88-30) K = 58 K
So,
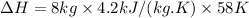 = 1948.8 kJ
= 1948.8 kJ
So, enthalpy of water at
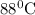 = enthalpy of water at
= enthalpy of water at
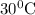 +
+
 = (1446 + 1948.8) kJ = 3394.8 kJ
= (1446 + 1948.8) kJ = 3394.8 kJ