Answer: This fuel will require 239230 years.
Step-by-step explanation:
All the radioactive reactions follow first order kinetics.
The equation used to calculate rate constant from given half life for first order kinetics:
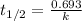
We are given:
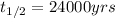
Putting values in above equation, we get:
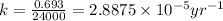
The equation used to calculate time period follows:
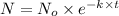
where,
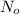 = initial mass of isotope = 10 kg = 10000 g (Conversion factor: 1 kg = 1000 g)
= initial mass of isotope = 10 kg = 10000 g (Conversion factor: 1 kg = 1000 g)
N = mass of the parent isotope left after the time = 10 g
t = time = ? years
k = rate constant =
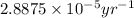
Putting values in above equation, we get:
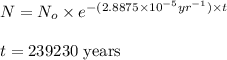
Hence, this fuel will require 239230 years.