Step-by-step explanation:
Total volume of the solution will be (10 ml + 10 ml) = 20 ml. As there are 1000 ml in 1 L. So, 20 ml will be equal to 0.02 L.
As molarity equals number of moles divided by volume in liter. Hence, calculate number of moles of
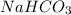 as follows.
as follows.
No. of moles =
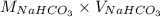
=
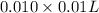
=
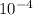 mol
mol
Hence, moles of
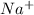 will also be equal to
will also be equal to
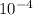 mol.
mol.
Now, calculate the no. of moles of
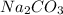 as follows.
as follows.
No. of moles =
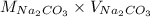
=
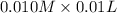
=
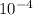 mol
mol
As there are 2
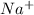 ions in 1 mole of
ions in 1 mole of
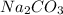 . Hence, in
. Hence, in
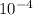 mol number of
mol number of
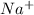 ions will be
ions will be
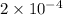 mol.
mol.
When both the solutions are mixed together then molarity of the solution will be calculated as follows.
Molarity of solution =
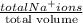
=
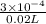
= 0.015 M
Thus, we can conclude that the molar concentration of Na (aq) in the given solution is 0.015 M.