Answer: Option (d) is the correct answer.
Step-by-step explanation:
Steps involved for the given reaction will be as follows.
Step 1:
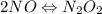 (fast)
(fast)
Rate expression for step 1 is as follows.
Rate = k
![[NO]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/degyd5c8ug7lahiab48ipwvlj63tp8bc8w.png)
Step 2:
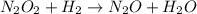
This step 2 is a slow step. Hence, it is a rate determining step.
Step 3.
 (fast)
(fast)
Here,
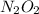 is intermediate in nature.
is intermediate in nature.
All the steps are bimolecular and it is a second order reaction. Also, there is no catalyst present in this reaction.
Thus, we can conclude that the statement step 1 is the rate determining step, concerning this mechanism is not directly supported by the information provided.