Step-by-step explanation:
Formula for energy value is as follows.
E =

where,
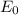 = 13.6
= 13.6
n = number of shell
So, at ground state energy of hydrogen atom will be as follows.
E =

=

= -13.6
Hence, for an ionized atom value of energy will be as follows.
E' =

or, E' =

Thus, we can conclude that total energy of the remaining electron in the ionized atom is
 .
.