Answer:
(a)
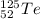
(b)
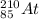
(c)
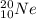
(d)
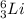
Step-by-step explanation:
Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
(a) the chalcogen with a mass number of 125
Chalcogens are the elements in the Group 16 in the Periodic Table. Tellurium has an average atomic mass of 127.6 u so it must be an isotope of it. The atomic number of Te is 52. The symbol is
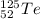 .
.
(b) the halogen whose longest-lived isotope is radioactive
Halogens are the elements in the Group 17 of the Periodic Table. The atom whose longest-lived isotope is radioactive is astatine, with a mass number = 210 and an atomic number = 85. The symbol is
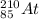 .
.
(c) the noble gas, used in lighting, with 10 electrons and 10 neutrons
The noble gas used in lighting is neon, with 10 protons (atomic number = 10), 10 electrons and 10 neutrons (mass number = 10 p⁺ + 10 n⁰ = 20). The symbol is
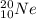 .
.
(d) the lightest alkali metal with three neutrons
Alkali metals are the elements in the Group 1 in the Periodic Table. The lightest one is lithium with 3 protons (atomic number = 3). The isotope with 3 neutrons has a mass number = 3 p⁺ + 3 n⁰ = 6. The symbol is
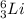 .
.