Step-by-step explanation:
The given data is as follows.
mass = 2.72 g
volume = 0.121

As the density is mass divided by volume.
Mathematically, Density =

Hence, putting the given values into the above formula and calculate the density as follows.
Density =

=
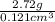
= 22.47
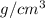
Thus, we can conclude that density of osmium is 22.47
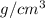 .
.