Step-by-step explanation:
As it is known that there are two types of properties. These are extensive and intensive.
Extensive properties : Properties that depend on the size or amount of system. For example, mass, volume etc.
Intensive properties : Properties that do not depend on the size or amount of system. For example, density, melting point, specific heat capacity etc.
On the basis of these properties water and ethanol are distinguished as follows.
- Density of water is 997 kg/
 whereas density of ethanol is 789 kg/
whereas density of ethanol is 789 kg/
 . Both these liquids can be separated by intensive properties.
. Both these liquids can be separated by intensive properties. - Melting point of water is zero degree celsius whereas melting point of ethanol is -114.1 degree celsius.
- Specific heat capacity of water is 4.184
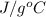 whereas specific heat capacity of ethanol is 2.46
whereas specific heat capacity of ethanol is 2.46
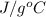 .
. - Mass of the given liquids cannot be differentiated because they will keep on changing depending on the quantity required. As mass is an extensive property, therefore, it is difficult to differentiate between the two liquids.
Thus, we can conclude that properties like density, melting point, specific heat capacity can help a chemist distinguish between ethanol and water.