Answer : The concentration of
 at equilibrium will be, 0.0086 M
at equilibrium will be, 0.0086 M
Explanation : Given,
Equilibrium constant =
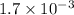
The balanced equilibrium reaction is,
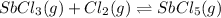
Initial conc. 0.0546 0.0546 0
At eqm. (0.0.546-x) (0.0.546-x) x
The expression of equilibrium constant for the reaction will be:
![K_c=([SbCl_5])/([SbCl_3][Cl_2])](https://img.qammunity.org/2020/formulas/chemistry/college/e80n4vryni4uy4mwnhoxlad4mexcr9fy7u.png)
For the given the value of
 will be,
will be,
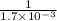
Now put all the values in this expression, we get :
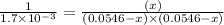
By solving the term 'x', we get:
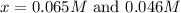
From the values of 'x' we conclude that, x = 0.065 can not be more than initial concentration. So, the value of 'x' which is equal to 0.065 is not consider.
So, x = 0.045 M
Thus, the concentration of
 at equilibrium =
at equilibrium =
![(0.0546-x)M=[0.0546-2(0.045)]M=0.0086M](https://img.qammunity.org/2020/formulas/chemistry/college/mvf586ndy1q8veuo1o7r73hv94rbmhrnoz.png)