Answer:
41.66 mL of 12.0 M sulfuric acid are needed.
Step-by-step explanation:
Concentration of sulfuric acid solution taken =
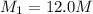
Volume of the 12.0 M Solution =

Concentration of required solution =
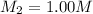
Volume of required 1.00 M solution =
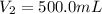
 (Dilution)
(Dilution)
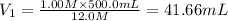
41.66 mL of 12.0 M sulfuric acid are needed.
The freezing point and the boiling point of a solvent when a non-volatile solute is dissolved in it decrease and increase respectively.