Answer: The correct answer is Option a.
Step-by-step explanation:
To calculate the Vant't Hoff factor, we use the equation for osmotic pressure, which is:
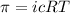
where,
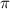 = osmotic pressure of the solution = 7.6 atm
= osmotic pressure of the solution = 7.6 atm
i = Van't hoff factor = ?
c = concentration of
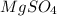 = 0.20 mol/L
= 0.20 mol/L
R = Gas constant =
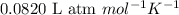
T = temperature of the solution =
![25^oC=[273+25]=298K](https://img.qammunity.org/2020/formulas/chemistry/college/6emvaajqo5qvucrhq2qn2dbo2gul9o60b4.png)
Putting values in above equation, we get:
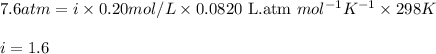
Hence, the correct answer is Option a.