Answer: 18.9 calories
Explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
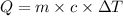
Q = Heat absorbed
m= mass of substance = 1.0 g = 0.001 kg
c = specific heat capacity of aluminium =
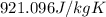
Initial temperature =
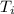 = 847 K
= 847 K
Final temperature =
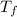 = 933 K
= 933 K
Change in temperature ,
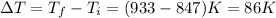
Putting in the values, we get:
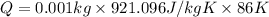
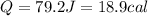 (1J=0.24cal)
(1J=0.24cal)
The number of calories required for this heat up process is 18.9 calories.