Answer:
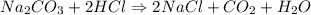
Option A is the Answer
Step-by-step explanation:
Balancing is making the number of atoms of each element same on both the sides (reactant and product side).
To find the number of atoms of each element we multiply coefficient × subscript
For example
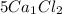 contains
contains
5 × 1 = 5 , Ca atoms and
5 × 2 = 10, Cl atoms
If there is a bracket in the chemical formula
For example
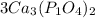 we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
So 3 × 3 = 9, Ca atoms
3 × 1 × 2 = 6, P atoms
3 × 4 × 2 = 24, O atoms are present.
A)
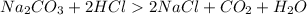
Reactant Side # of atoms of each element Product Side
2 Na 2
1 C 1
3 O 3
2 H 2
2 Cl 2
Balanced!!!!
B)
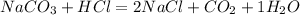
(Chemical formula given for Sodium carbonate is not correct)
Na2CO3 is the correct formula
C)
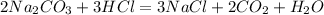
(Not balanced correctly)
Reactant Side # of atoms of each element Product Side
4 Na 3
2 C 2
6 O 5
3 H 2
3 Cl 3
Not same on both the sides
Hence UnBalanced
D)
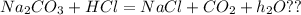
Unbalanced