Answer : The correct option is, Rust formation is an exothermic reaction because it releases energy.
Explanation :
Endothermic reaction : It is a type of chemical reaction in which the energy is absorbed from the surrounding.
Exothermic reaction : It is a type of chemical reaction in which the energy is released into the surrounding.
Iron rusting : It is a chemical process in which an iron nail react with water and oxygen to give iron oxide as a product. Rusting of iron is an oxidation-reduction reaction.
The rust formation is represented as:
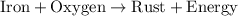
From the this we conclude that, the energy is released during the rusting formation. That means, it is an exothermic process.
Hence, the correct option is, Rust formation is an exothermic reaction because it releases energy.