Given:
refractive index of pure hexane,
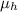 = 1.375
= 1.375
refractive index of pure hexane,
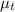 = 1.497
= 1.497
refractive index of mixture,
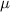 = 1.401
= 1.401
Formula used:
As refractive index here, behaves like a colligative prop. then using the following formula:
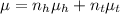 (1)
(1)
where,
 = hexane mole fraction
= hexane mole fraction
 = toulene mole fraction
= toulene mole fraction
 +
+
 = 1 (2)
= 1 (2)
Solution:
Now, using given formula from eqn (1)
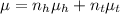
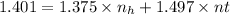 (3)
(3)
Multiply eqn (2) by 1.375, we get:
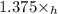 + 1.375\times
+ 1.375\times
 = 1.375 (4)
= 1.375 (4)
Now, solving eqn (3) and (4):
 = 0.213
= 0.213
Substituting the value of
 = 0.213 in eqn (1), we get:
= 0.213 in eqn (1), we get:
 = 1 - 0.213
= 1 - 0.213
 = 0.787
= 0.787
Therefore, mole fraction of hexane in the sample is
 = 0.787
= 0.787