Step-by-step explanation:
The given reaction is as follows.
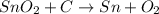
a). Molar mass of
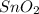 is [(mass of Sn) + (2 × mass of O)].
is [(mass of Sn) + (2 × mass of O)].
Therefore, molar mass of
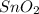 = (118.7 + 2 × 16) g/mol = 150.7 g/mol
= (118.7 + 2 × 16) g/mol = 150.7 g/mol
Since, it is known that number of moles equal mass divided by molar mass. So, moles of Sn will be calculated as follows.
No. of moles =
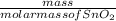
=
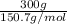
= 2.53 mol
As it is given that 1 mole of
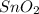 produces 1 moles of Sn and 1 moles of
produces 1 moles of Sn and 1 moles of
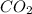 . Hence, 2.53 moles of
. Hence, 2.53 moles of
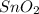 will also produce 2.53 moles of Sn and 2.53 moles of
will also produce 2.53 moles of Sn and 2.53 moles of
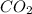 .
.
Volume of 1 mole of
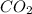 at STP is 22.4 L. Therefore, volume of 2.53 moles of
at STP is 22.4 L. Therefore, volume of 2.53 moles of
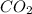 will be calculated as follows.
will be calculated as follows.
2.53 × 22.5 L = 56.67 L
Hence, 56.67 L of
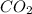 are produced if 300.0 grams of tin are produced at STP.
are produced if 300.0 grams of tin are produced at STP.
b). Mass of tin is given as 1800.0 g. So, number of moles will be calculated as follows.
No. of moles =
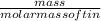
=
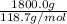
= 15.2 moles
As 15.2 moles of
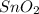 produces 15.2 moles of Sn. Therefore, weight of
produces 15.2 moles of Sn. Therefore, weight of
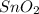 will be calculated as follows.
will be calculated as follows.
Mass = no. of moles × molar mass of
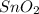
= 15.2 moles × 150.7 g/mol
= 2290.64 g
Hence, 2290.64 grams of
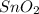 are required to produce 1800.0 grams of tin.
are required to produce 1800.0 grams of tin.
c). Mass of carbon given is 100.0 grams.
No. of moles =
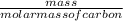
=
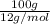
= 8.33 moles
As, 1 mole of carbon is produced by 1 mole of tin. So, 8.33 mole of carbon will be produced by 8.33 moles of tin.
Therefore, calculate mass of tin produced as follows.
Mass = no. of moles × molar mass of Sn
= 8.33 moles × 118.7 g/mol
= 988.8 g
Hence, 988.8 grams of tin will be produced per 100 grams of carbon used.