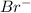Answer:
Lattice Energy
Step-by-step explanation:
Melting point is the temperature at which a solid changes into a liquid.
The process is called as Melting.
Lattice energy is the energy required by an ionic compound in order to overcome the electrostatic force which is existing between the oppositely charged ions.
Ionic compound which has a strong electrostatic force will have high melting point .
Energy required to break the ionic bond or the Lattice array is more.
Lattice energy depends on two main factors
1) charge of the ions and
2) size of the ions
Lattice energy increases with the increase in charge.
For example Lattice energy of MgO is larger than NaCl.
Mg and O have 2+ and 2- charges respectively
Na and Cl have 1+ and 1- charges.
Lattice energy increases with decrease in size of the ions.
For example NaCl Lattice energy is larger than KBr.
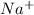 is smaller than
is smaller than
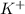 ion
ion
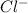 is smaller than
is smaller than