Step-by-step explanation:
According to the ideal gas equation, PV = nRT.
So, V =
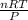 ......... (1)
......... (1)
Since, it is given that volume of the gas is increases. So, change in volume will be as follows.
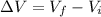
Hence, equation (1) will become as follows.
 =
=
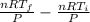
 = nR
= nR

Therefore, work done on the gas will be given as follows.
W = - P

=
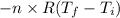
=
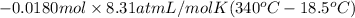
= 48.09 J
Thus, we can conclude that work done on the gas is 48.09 J.