Step-by-step explanation:
According to the water table, the value of enthalpy of evaporation at a temperature of
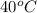 is 2406.0 kJ/kg.
is 2406.0 kJ/kg.
Hence, we will calculate the rate of heat transfer by using the formula as follows.
Q =
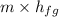
where, m = mass
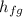 = enthalpy of evaporation
= enthalpy of evaporation
Putting the given values into the above formula as follows.
Q =
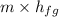
=
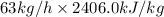
= 151578 kJ/h
or, =
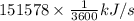
= 42.105 kW
Thus, we can conclude that rate of heat transfer from the steam to the cooling water flowing through the pipe is 42.105 kW.