Answer:
Step-by-step explanation:
The half reactions are:
Mg
 → Mg²⁺
→ Mg²⁺
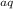 + 2e⁻
+ 2e⁻
2H⁺
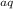 + 2e⁻ → H₂
+ 2e⁻ → H₂

The first equation is an oxidation half while the second is a reduction half. To get the overall reaction equation, we simply combine the two equations. The reactants combines at the left hand side with the products comes together at the right hand side.
Mg
 + 2H⁺
+ 2H⁺
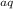 + 2e⁻ → Mg²⁺
+ 2e⁻ → Mg²⁺
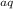 + 2e⁻ + H₂
+ 2e⁻ + H₂

Now we cancel out the species that appear on both sides:
Mg
 + 2H⁺
+ 2H⁺
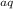 → Mg²⁺
→ Mg²⁺
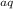 + H₂
+ H₂
