Answer:
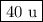
Step-by-step explanation:
The atomic mass is the weighted average of the atomic masses of each isotope.
In a weighted average, we multiply each value by a number representing its relative importance.
In this problem, the percent abundance represents the relative importance of each isotope.
Data:
K-39 = 23 %
K-40 = 48 %
Calculations:
(a) Calculate % K-41
K-41 = 100 - 23 - 48 = 29 %
(b) Calculate atomic mass of sample
