Answer: The equilibrium concentration of
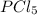 is 1.285 M.
is 1.285 M.
Step-by-step explanation:
The chemical equation for the decomposition of phosphorus pentachloride follows:
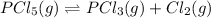
The expression for equilibrium constant is given as:
![K_c=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2020/formulas/chemistry/middle-school/gpkvotujabdxursdhkqb051q385dj25ify.png)
We are given:
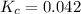
![[PCl_3]=0.18M](https://img.qammunity.org/2020/formulas/chemistry/high-school/jq9shyssrjykl7raumlgj7lj5ntgpfgftr.png)
![[Cl_2]=0.30M](https://img.qammunity.org/2020/formulas/chemistry/high-school/rrxithaz9xzpd1euzd97mwxg139iyd1u6r.png)
The concentration of solid substances are taken to be 1. Thus, they do not appear in the equilibrium constant expression.
Putting values in above equation, we get:
![0.042=(0.18* 0.30)/([PCl_5])](https://img.qammunity.org/2020/formulas/chemistry/high-school/l0igomd8r70bgug8seye1fh8ddicwlo6vk.png)
![[PCl_5]=1.285](https://img.qammunity.org/2020/formulas/chemistry/high-school/tq0vum0yiqr0laelqegqpt2i3vq5ir82ih.png)
Hence, the equilibrium concentration of
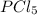 is 1.285 M.
is 1.285 M.