Answer:
(a)
P₂ = 7.13 atm
(b)
T₂ = 157.14 K
Step-by-step explanation:
(a)
V₁ = initial volume = 3.7 L = 3.7 x 10⁻³ m³
V₂ = final volume = 0.85 L = 0.85 x 10⁻³ m³
P₁ = Initial Pressure of the gas = 0.91 atm = 0.91 x 101325 = 92205.75 Pa
P₂ = Final Pressure of the gas = ?
Using the equation
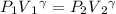
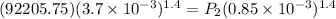
 = 722860 Pa
= 722860 Pa
 = 7.13 atm
= 7.13 atm
(b)
T₁ = initial temperature =283 K
T₂ = Final temperature = ?
using the equation
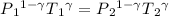
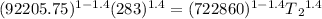
T₂ = 157.14 K