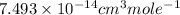Answer : The rate constant for this reaction at 384.7 K is,
Explanation :
The relation between frequency factor, rate constant and activation energy for a chemical reaction is,
![k=A* e^{[(-Ea)/(RT)]}](https://img.qammunity.org/2020/formulas/physics/college/6e8se7ppby5jfwg8w7kpa6jn6tue943m2u.png)
where,
k = rate constant = ?
A = frequency factor =
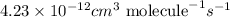
Ea = activation energy = 12.9 kJ/mol
T = temperature = 384.7 K
Now put all the given values in this formula, we get:
![k=4.23* 10^(-12)cm^3\text{ molecule}^(-1)s^(-1)* e^{[(-12.9kJ/mol)/((8.314J/mole.K)* (384.7K))]}](https://img.qammunity.org/2020/formulas/physics/college/vjma7eh1nss3n2v7ac8nagdl33wkbjx82p.png)
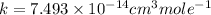
Therefore, the rate constant for this reaction at 384.7 K is,