Answer:
34.17°C
Step-by-step explanation:
Given:
mass of metal block = 125 g
initial temperature
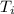 = 93.2°C
= 93.2°C
We know
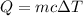 ..................(1)
..................(1)
Q= Quantity of heat
m = mass of the substance
c = specific heat capacity
c = 4.19 for H₂O in
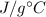
 = change in temperature
= change in temperature
Now
The heat lost by metal = The heat gained by the metal
Heat lost by metal =
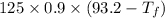
Heat gained by the water =
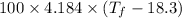
thus, we have
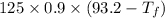 =
=
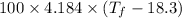
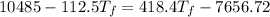
⇒
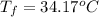
Therefore, the final temperature will be = 34.17°C