Answer:
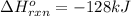
Step-by-step explanation:
Hello,
In this case, the standard enthalpy of reaction could be computed via the bond energies when both broken or made as shown below:
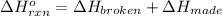
In this manner, we infer that at the reactants for ethene,
 a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
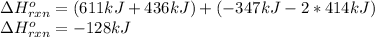
Best regards.