Answer:
a)905,89 atm of pressure will be generated at 298K.
b)The sap of tree can rise upto 9,357.84 meters.
Step-by-step explanation:
a)
Effective concentration of sap = c = 37 M
Osmotic pressure generate at 298K =
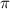
Temperature ,T = 298 K
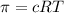
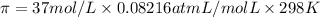
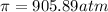
905,89 atm of pressure will be generated at 298K across the endodermis root membrane.
b)
Given that 1.0 at of pressure raises the volume of water upto height of 10.33 m
Then 905.89 atm of pressure will raise the height of water upto:
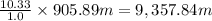
The sap of the tree can rise upto 9,357.84 meter.