Answer:
36.92 mg of oxygen required for bio-degradation.
Step-by-step explanation:
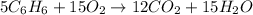
Mass of benzene = 30 mg = 0.03 g (1000 mg = 1 g )
Moles benzene =
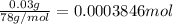
According to reaction 5 moles of benzene reacts with 15 moles of oxygen gas.
Then 0.0003846 mol of benzene will react with:
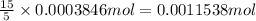 of oxygen gas
of oxygen gas
Mass of 0.0011538 moles of oxygen gas:
0.0011538 mol × 32 g/mol = 0.03692 g = 36.92 mg
36.92 mg of oxygen required for bio-degradation.