Answer:
The number of atoms in the gemstones of that tiara is
 .
.
Step-by-step explanation:
Number of diamonds ion tiara = 888
Mass of each diamond = 1.0 carat = 0.200 g (given)
Mass of 888 diamonds in tiara:
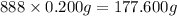
Given that diamond is a form of carbon.
Atomic mass of carbon atom = 12 g/mol
Moles of 77.600 g of carbon =

Number of atoms of carbon 14.800 moles:
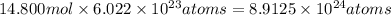
The number of atoms in the gemstones of that tiara is
 .
.