One equation for the number of moles in a compound:
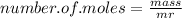
(note: Mr is the combined molecular mass of the compound.)
__________________________________
On the periodic table:
Lithium (Li) has a molecular mass of around 7
Fluorine (F) has a molecular mass of around 19.
-------------------------------
So the total Mr of Lithium Fluoride (LiF) is:
7 + 19 = 26
________________________________________
Now to work out the number of moles, we just divide the mass (in grams) of the compound by the Mr of the compound (as in the formula):
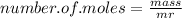
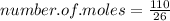
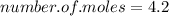
_______________________________
Answer:
A) 4.2